15. 5. 2024
Dr Petr Cígler and his collaborators are working on refining molecular systems for transporting ribonucleic acid (RNA) molecules into cells. The question of how to effectively deliver RNA to a designated place in the body in order to silence a malfunctioning gene is one of the greatest challenges of the rapidly developing field of gene medicine. Now, researchers from IOCB Prague have taken yet another significant step towards achieving this goal. They have described in detail the preparation of a novel composite vector nanomaterial for transporting RNA, in which they focused mainly on ensuring its non-toxicity to cells. The reason is that the harmfulness of hitherto known nucleic acid vectors presents a serious obstacle to the expansion of gene therapy. A new article on the topic has now been published in the scientific journal Advanced Functional Materials.
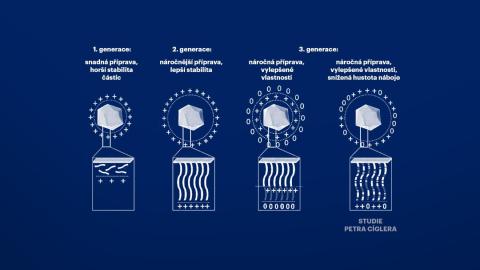
Petr Cígler and his colleagues worked with the assumption that the toxicity of nucleic acid vectors is caused by an excessive accumulation of positive charges along the chains of carrier polymers that bind nucleic acids. Therefore, they systematically diluted this positive charge with uncharged monomers and found that its slight dilution did not impair the ability of the system to transport nucleic acids. In their thorough study, they also describe the circumstances under which new materials can be prepared for use in gene medicine. The researchers paid close attention to the conditions in which these substances bind nucleic acids most effectively and in what stoichiometric proportion.
Cells can regulate the translation of genes from mRNA to proteins in several ways. One of them is to silence a gene with the help of an siRNA (small interfering RNA) molecule. This blocks the production of the target protein, which is especially useful if the gene encoding is mutated or otherwise defective. In addition, it is possible to intervene if the innate mechanisms of translation regulation are impaired. In such a case, too much of a protein is produced in the body, which is detrimental to its health.
‘Gene therapy is not only highly effective, but also acutely targeted. Its biggest advantage lies in the fact that it accurately targets individual mutations or disorders without the danger of missing the mark. Using siRNA, it is possible to hit even a single mutated “letter” of a gene,’ says Marek Kindermann, the first author of the study, listing the main advantages of gene therapy. Currently, five drugs based on siRNA are approved for worldwide use, and there are about a dozen more on the ‘waiting list’.
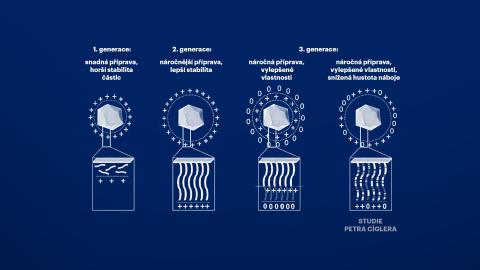
Scheme of various engineering strategies for the production of cationic polymer-coated NPs
The therapeutic use of siRNA is hindered by the fact that siRNA molecules are unstable and therefore break down very quickly in the body. Experts around the world are therefore addressing the problem of how to deliver siRNA to a designated location and allow it to do its job, which is to stop or limit the production of a certain undesirable protein by silencing the gene for it. This task was also taken up by Petr Cígler's team at IOCB Prague, together with colleagues from the Institute of Microbiology of the Czech Academy of Sciences, led by the head of the Nanomedicine group, Dr Veronika Benson. And the results that they have achieved are truly excellent.
‘Each transport system is designed to meet two basic objectives: First of all, it has the task of protecting the molecule from decaying, and secondly, it must ensure its transport into the cell so that it reaches the cytosol and fulfills its mission as a drug there,’ explains Petr Cígler, adding: ‘In our study, we deal with the properties of the transport system in great detail. We descend all the way down to the level of structural minutiae of molecules that interact with nucleic acids. We describe the conditions necessary for siRNA to successfully bind to the transport nanosystem and then reach the intracellular space.’

Dr. Petr Cígler, head of the senior research group Synthetic Nanochemistry at IOCB Prague (Photo: Tomáš Belloň / IOCB Prague)
Researchers from IOCB Prague are thus paving the way for the use of particles referred to as non-viral vectors, which do not utilize viruses to carry RNA. They have placed the entire transport system, including the nucleic acid, on the surface of diamond nanoparticles. These are markedly stable carrier particles, which also emit a special type of fluorescence. This makes it possible to track their journey through tissues and monitor how they behave inside cells. One complication is that it is difficult for the body to get rid of nanodiamonds, so this method of gene therapy is suitable mainly for the treatment of hard-to-heal superficial wounds. It is for these types of therapy, with a focus on healing leg ulcers in patients with diabetes, that the new transport nanomaterials, presented in this and other works by the team of Dr Cígler and collaborators, are intended.
Their latest in-depth study was also made possible thanks to the AMULET project, which focuses on the development of multi-scale nanomaterials and brings together eight partners led by the Jaroslav Heyrovský Institute of Physical Chemistry. The AMULET project has received financial support from the Operational Programme Johannes Amos Comenius, administered by the Ministry of Education, Youth and Sports of the Czech Republic, in the category of Cutting-Edge Research.
Original article: Kindermann M., Neburkova J., Neuhoferova E., Majer J., Stejfova M., Benson V., Cigler P. Design Rules for the Nano-Bio Interface of Nanodiamonds: Implications for siRNA Vectorization. Advanced Functional Materials, 2314088 (2024). https://doi.org/10.1002/adfm.202314088
Video: https://youtu.be/fU6xHhE4s9w